Our Services
professional technology Empowers Full Life Cycle of Clinical Trial Phase Ⅰ, Ⅱ, Ⅲ, Ⅳ
Our Advantages
We are professional and provide professional services

Study Sites 50+

Clinical Study Projects 100+

Multi-Regional Clinical Trial (MRCT) Experience

CTMS (Clinical Trial Management System)

SOPs (Standard Operating Procedures)
About Us
Shanghai Chengtai Pharmaceutical Technology Co., Ltd.

Shanghai Chengtai Pharmaceutical Technology Co., Ltd. (CHAINTECH) is a renowned contract research organization (CRO) specializing in pharmaceutical clinical trials in China, with a particular focus on prophylactic vaccines. For over a decade, the company has demonstrated unwavering commitment to delivering efficient and high-quality services in vaccine clinical research.
With a proficient and experienced team, CHAINTECH has successfully completed over 100 clinical research projects involving various vaccines. The company has played a pivotal role in facilitating the licensing and market authorization of 12 vaccine products. More than 30 clinical studies overseen by CHAINTECH have passed pre-licensure inspections from National Medical Products Administration (NMPA).
CHAINTECH has forged extensive partnerships with key research institutions such as Provincial Centers for Disease Control and Prevention across China, including Henan, Yunnan, Shanxi, Hunan, Hubei, Hebei, and Jiangsu. Their collaborative efforts span over 50 study sites, demonstrating its proficiency in site organization and project implementation.
Moreover, CHAINTECH has the capability to conduct large-scale multi-regional clinical trials (MRCT) with a proven track record. The company has developed a proprietary computerized system covering the full lifecycle of a vaccine clinical trial with the highest success rate of passing pre-licensure inspections by NMPA. Leveraging this robust system, CHAINTECH provides tailored solutions to meet the diverse needs and settings of clinical trial stakeholders.
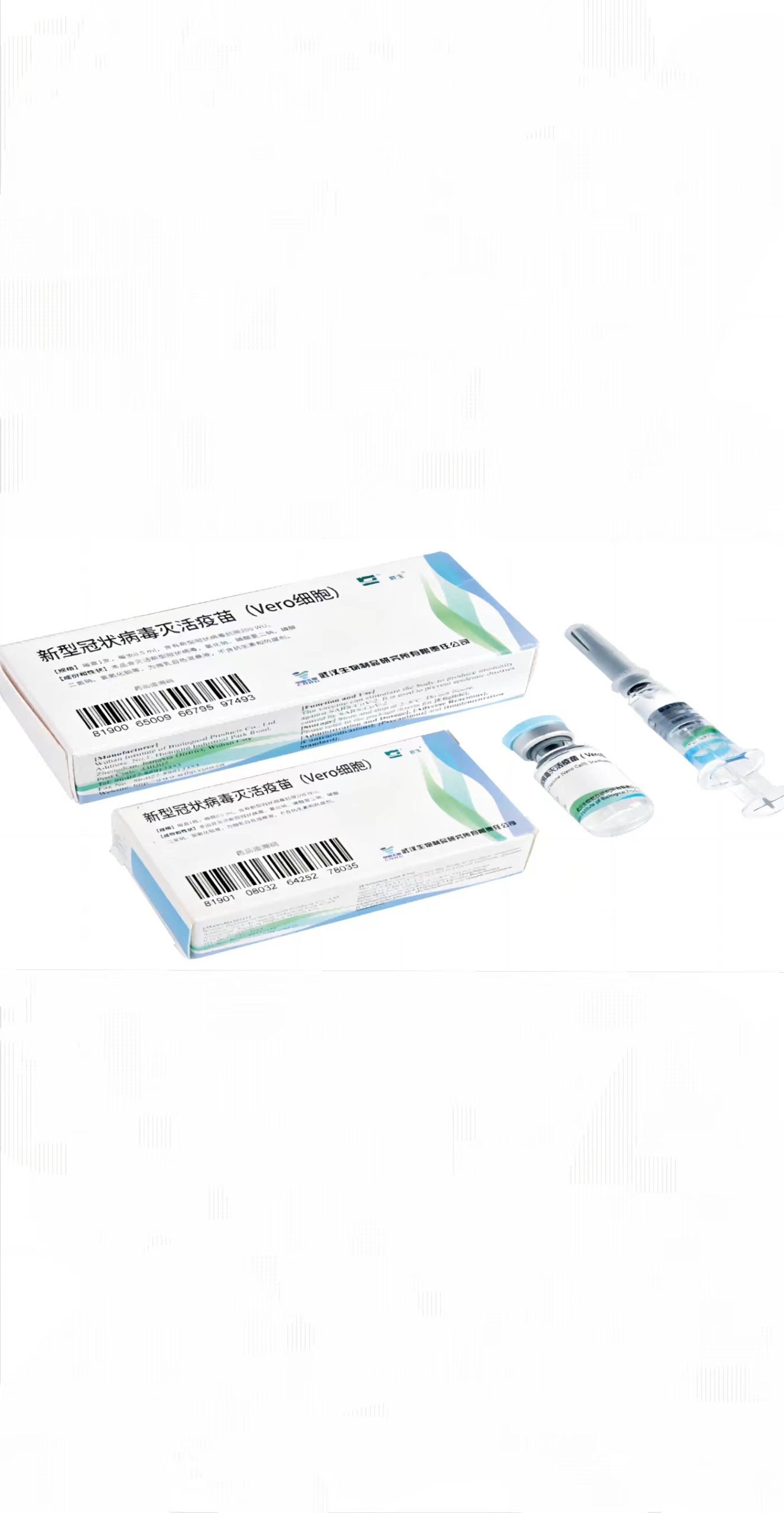
Displaying Products Successfully Serviced for Market Launch
Project Experience 100+ In-depth, Full Service, Quality First
News
Fresh information, warm service
Our Customers
Collaboration is the Choice of the Era, Collaboration is the Foundation of Success
-

Sinopharm CNBG(China National Biotec Group Company Limited)
-

SINOVAC(Sinovac Biotech Ltd.)
-

Sanofi
-

Walvax(Walvax Biotechnology Co., Ltd.)
-

CanSino(CanSino Biologics Inc.)
-

CDBIO (Chengda Biotechnology)
-

MICROVAC
-

Recbio(Jiangsu Recbio Technology Co., Ltd.)
-

Abzymo (Abzymo Diagnostic Technology Inc.)
-

Maxvax (Maxvax Biotechnology)
-

Ab&B Bio(Ab&B Bio-Tech CO.,LTD.JS)
-

HUALAN BIO
-

Adimmune
-

IMBCAM (Institute Of Medical Biology Chinese Academy Of Medical Sciences Peking Union Medical College)
-

Olymvax(Chengdu Olymvax Biopharmaceuticals Inc.)
















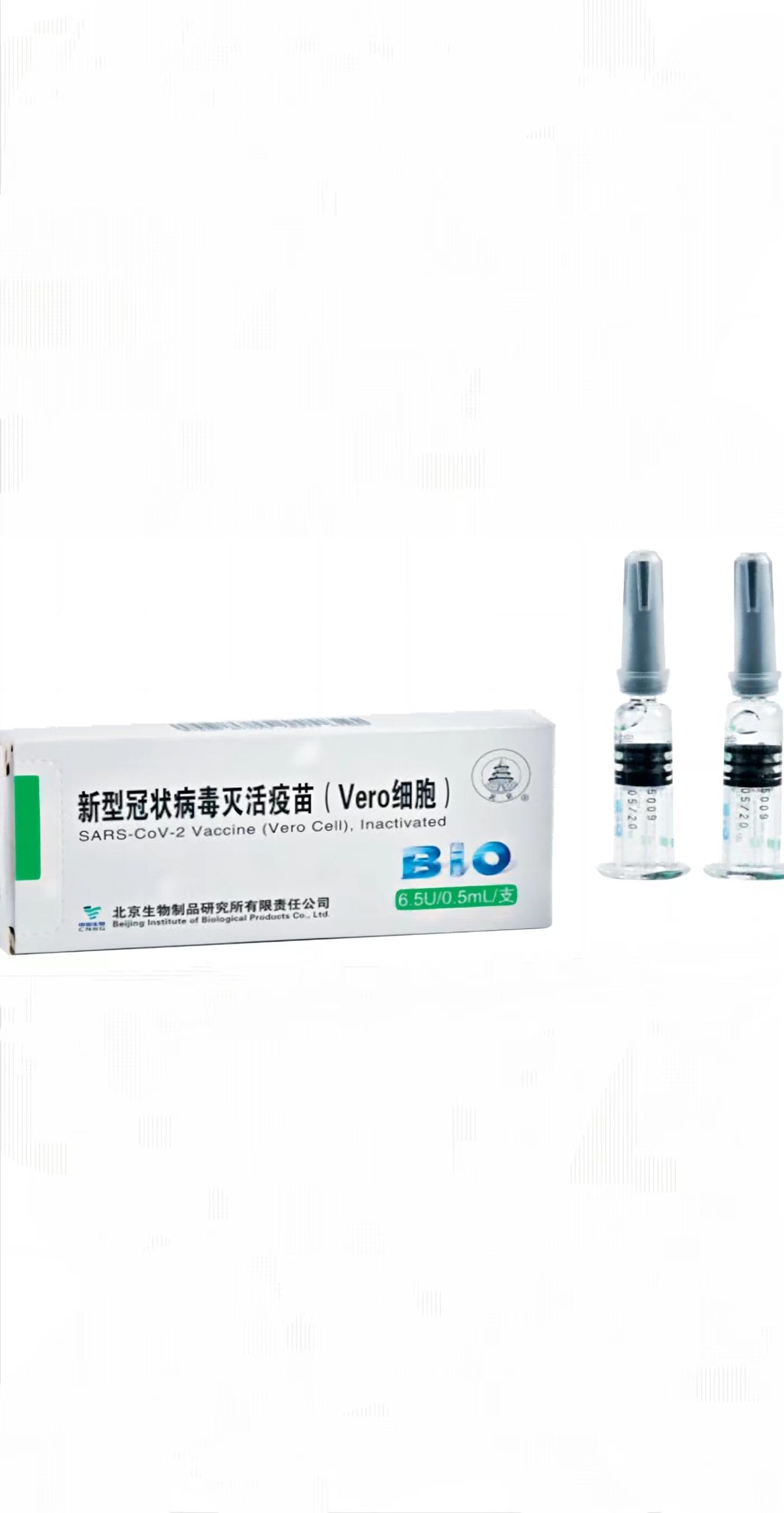
 Sinopharm-BIBP SARS-CoV-2 Vaccine (VeroCell), Inactivated
Sinopharm-BIBP SARS-CoV-2 Vaccine (VeroCell), Inactivated